Cracking Decane Chemical Equation
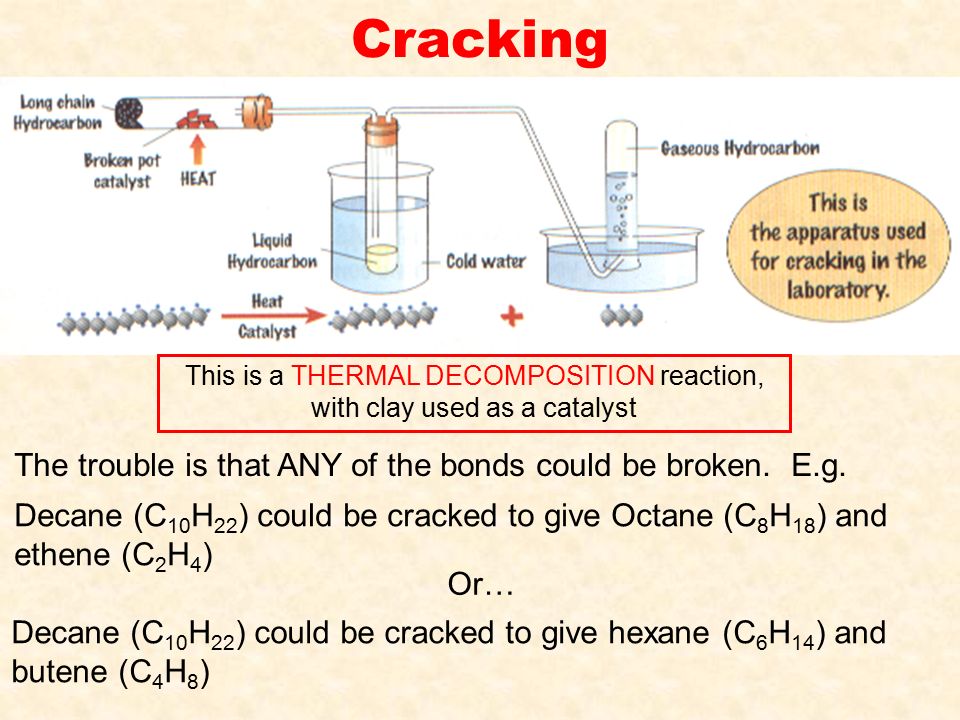
CRACKING HYDROCARBONS - useful products Doc Brown's GCSE/IGCSE/O Level KS4 science–CHEMISTRY Revision Notes Oil, useful products, environmental problems, introduction to organic chemistry 6. Cracking – a problem of supply and demand, other products – Cracking is a thermal decomposition process by which large alkane hydrocarbon molecules are broken down by passing them over a heated catalyst at high pressure. The products are smaller alkanes used for fuels (e. Download Free Instruction Manual For Monster Jam Pc Games. g. Petrol or diesel) and alkenes which are used to make polymers–plastics and other important compounds. There are two good economic reasons for cracking oil fractions – (i) there isn't enough of fuels like petrol or diesel in the original crude oil and (ii) alkenes are NOT found in oil, so must be manufactured from oil. Either way, it means the vast majority of crude oil can be turned into useful products. These notes on cracking alkanes to make alkenes for plastics and other products from cracking like petrol and diesel fuels are designed to meet the highest standards of knowledge and understanding required for students/pupils doing GCSE chemistry, IGCSE chemistry, O Level chemistry and KS4 science courses. These revision notes on cracking hydrocarbons like alkanes in the petrochemical industry and why the cracking reaction is so important should prove useful for the NEW AQA GCSE chemistry, Edexcel GCSE chemistry & OCR GCSE chemistry (Gateway & 21st Century) GCSE (9–1), (9-5) & (5-1) science courses.